The study of science is an important part of a child’s education and can be exciting and engaging when presented in a way that captures their interest. One of the key topics in science that kids learn about is the states of matter: solids, liquids, and gases. Understanding these states of matter is crucial to understanding the world around us, as everything we see and touch is made up of one of these three forms.
In this blog post, we will explore the magic of matter and provide a kid-friendly guide to understanding the properties of solids, liquids, and gases. We will dive into how these states of matter interact with each other and with heat to change from one form to another. We will also discuss real-world examples of these states of matter, describing how they are used in our everyday lives. By presenting this material in an engaging, easy-to-understand way, we hope to spark your child’s curiosity and help them gain a deeper understanding of the science behind matter.
Click the link for a video lesson on the phases of matter: solid liquid gas examples for kids
1. Matter exists in three main states: solids, liquids, and gases.

Matter exists in three primary states: solids, liquids, and gases. These three states refer to the ways in which matter can behave, based on the movement of its particles. In solids, the particles are arranged closely together and do not have much movement. Liquids, on the other hand, have particles that are farther apart and can move more freely, allowing them to flow and take the shape of their container while maintaining a fixed volume. Gases have particles that are even farther apart than those in liquids and are in constant random motion, resulting in a fluid state that fills the space that it occupies without a fixed shape or volume. By understanding these states of matter, we can gain a better understanding of the world around us, from ice melting to steam rising from a hot cup of cocoa, as well as how and why materials can change from one state to another with temperature and pressure.
2. Solids are objects that have a definite shape and volume.
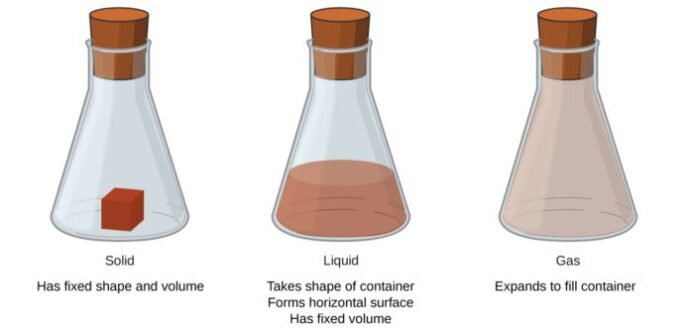
Solids are objects that have a definite shape and volume, which means they maintain their shape and take up a certain amount of space. Solids are made up of atoms and molecules that are tightly packed together and held in a fixed position by strong forces known as intermolecular forces. These atoms and molecules in solids vibrate in place, but they do not move freely like in liquids and gases. The arrangement of particles in a solid determines its properties and characteristics such as hardness, density, and brittleness.
3. Liquids take the shape of their container and have a definite volume
Liquids take the shape of their container and have a definite volume, like water or juice. This means that they will fill the space that is available to them, taking on the shape of the container in which they are poured. Liquids, unlike solids, do not have a particular shape but instead flow and conform to their environment. Similarly, their volume remains constant, which means that they cannot be compressed or expanded like gases.
4. Gases have no definite shape or volume and fill up the space around them.
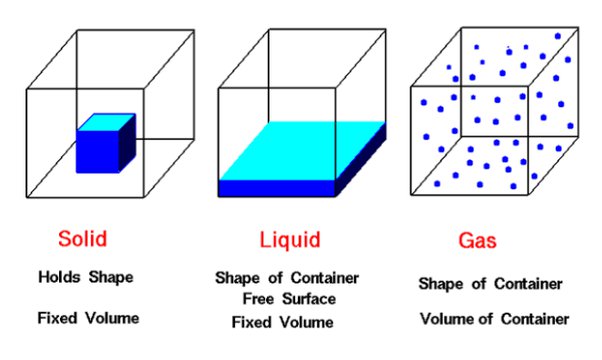
Unlike solids and liquids, the molecules in gases have no definite shape or volume, and they can move freely and fill up any space available to them. Just think about the air we breathe – it has no shape or volume and takes the shape of its container. This makes gases very useful in various applications, such as fueling cars, filling up balloons, powering turbines, and much more.
5. Matter can change from one state to another.
Did you know that matter can also change from one state to another through a process called phase change? One example of phase change is when ice melts into water. This happens when energy in the form of heat is added to the ice, causing its particles to gain energy and become more active. As they move around more freely, the bonds that hold them together weaken, and the ice melts into a liquid. Other examples of phase change include boiling water to turn it into steam, or freezing water to turn it into ice. These changes may seem like magic, but they actually follow the rules of science and can be explained through the study of matter and its properties.
6. The behavior of matter is affected by temperature and pressure.
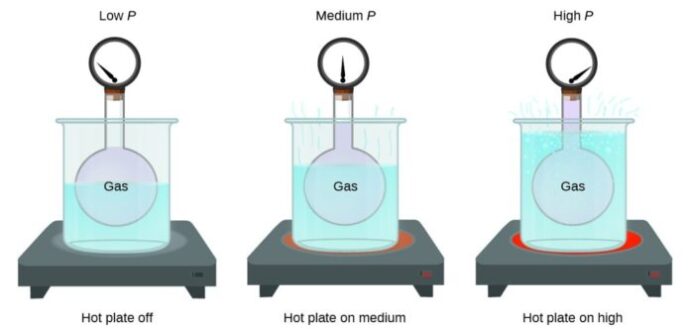
When we talk about temperature, we’re referring to the amount of heat something has. As we add heat to matter, the particles inside start to move faster and farther apart, turning solids into liquids and liquids into gases. On the other hand, lowering the temperature causes the particles to slow down and move closer together, turning gases into liquids and liquids into solids. Pressure also affects the behavior of matter. When we apply pressure, we are squeezing the particles together, causing them to become more tightly packed. Conversely, if we decrease the pressure, the particles will have more space to move around, causing the matter to expand.
7. Understanding the properties of matter is important in fields like chemistry and physics.
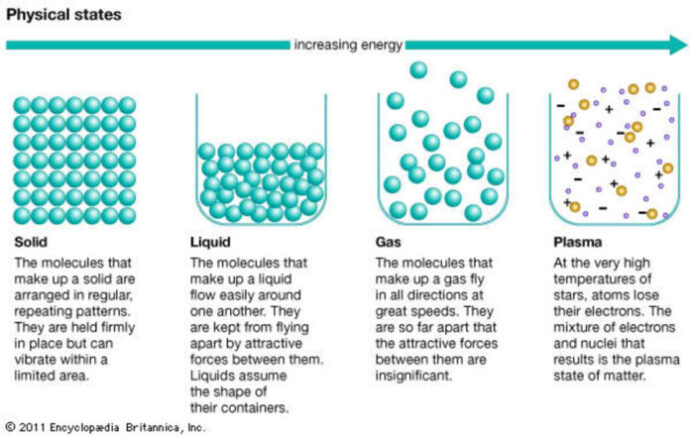
Understanding the properties of matter is key in fields like chemistry and physics. Chemistry is the study of the interactions between different types of matter, while physics focuses on the properties and behavior of substances and energy. Knowing the properties of solids, liquids, and gases helps scientists and engineers design and create new materials, as well as develop innovative technologies. For instance, understanding the properties of matter is critical in developing new drugs, manufacturing new materials, and designing new technologies.
In conclusion, matter is a fascinating topic with many properties and behaviors to explore. We now know that solids have definite shapes and volumes, liquids take the shape of their container but maintain a constant volume, gases fill up any space available to them due to having no definite shape or volume, temperature and pressure can affect how particles move around in matter, and understanding these concepts are important for fields like chemistry and physics. With this knowledge at our disposal, we can make new discoveries about the world around us every day!















